|
Year of acquisition |
2018-2020 |
|
Financing |
OP VVV CORE FACILITIES CZ.02.1.01/0.0/0.0/16_017/0002515 |
|
Responsible Person |
prof. Ing. Zdeněk Fiala, CSc. |
Every human activity can potentially pose risks to humans and the environment under certain circumstances. When these risks increase and accumulate, it is necessary to implement measures to reduce the risk level to a socially acceptable standard. However, it is first necessary to characterize the potential risk based on the identification and characterization of hazards and exposure assessment. As part of risk identification, it is also necessary to assess the ability of the risk factor to cause damage to the genetic information of cells, i.e., to act genotoxically.
The term genotoxicity refers to substances, factors, or processes that damage the structure of DNA, alter the informational content of the genome and the transfer of information, affect DNA segregation, or inhibit its replication. The process of genotoxicity largely overlaps with the process of mutagenicity, which is characterized by sudden, disordered changes in genetic information transmitted to subsequent generations of cells. Substances (factors) labeled as mutagens induce locally confined lesions in the DNA structure, which, if not removed by cellular repair systems, can be fixed into stable mutations during DNA replication. Mutations can occur either within individual genes (gene mutations) or result in chromosome damage (chromosomal mutations). Chromosomal changes can affect either the structure or number of chromosomes. For these reasons, mutagenicity tests can be considered genotoxicity tests.
Testing carcinogenic effects on animals or analyzing carcinogenic potential through epidemiological studies requires high costs and usually takes years. Short-term genotoxicity tests allow for a preliminary screening of several hundred thousand existing and newly produced (and used) substances, identifying those that signal potential carcinogenicity through their genotoxic effects. However, it is important to emphasize that, in addition to genotoxic mechanisms of carcinogenesis (where the first step in the process is induced by DNA damage through mutational changes), there are also non-genotoxic (epigenetic) mechanisms that cannot be identified by short-term genotoxicity tests.
The advantage of short-term genotoxicity tests is their potential for quickly detecting the presence of genotoxic effects and, depending on the type of test used, possibly also the mechanism of these effects. Based on the relationship between genotoxic and carcinogenic mechanisms of action, identifying a genotoxic effect can help pinpoint substances (factors) that need attention as potential carcinogens and should be further investigated in in vivo tests and epidemiological studies.
One of the significant short-term genotoxicity tests is the "Comet assay" (CA), which is used to detect breaks in cell nuclei. This versatile, relatively simple, and sensitive method can, depending on the variant, detect single and double-strand DNA breaks, alkali-labile sites (apurinic/apyrimidinic sites), incomplete DNA repair, as well as oxidized purine or pyrimidine bases, cross-links, and apoptotic cells. DNA breaks detectable by CA can be repaired in the cell, be lethal to the cell, or be fixed in the cell, resulting in a permanent mutation. DNA damage can be detected by CA in suspensions of yeast, protozoal, plant, and animal cells (both invertebrates and vertebrates), in both dividing and non-dividing cells.

Photo 1: (S) Setup for comet assay analysis (fluorescence microscope and PC with analysis software) with an example of measurements.

Photo 2: Biohazard box with laminar flow featuring two workstations, allowing two students to work simultaneously.
During the CA, cells are placed in an agarose gel. After lysing the cell and nuclear membranes in a salt solution, DNA fragments migrate out of the nucleus, more precisely from the nucleoid ("head") to the "tail" due to electrophoresis, creating the characteristic comet image after visualization with a suitable dye, which gives the method its name. The more fragmented the DNA, the larger and longer the "tail." The fragmented DNA, due to its negative charge, moves towards the anode.
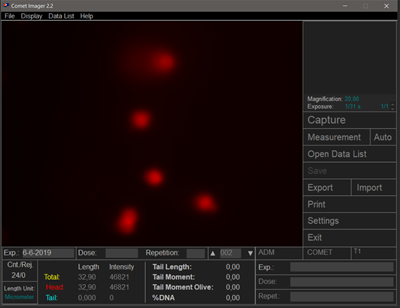
Photo 3: Example of "comets" from a comet assay test.
The CA assesses the extent of DNA damage caused by genotoxic substances present in the environmental and occupational settings and the ability to repair damaged DNA. The method is used in in vitro, in vivo, and epidemiological studies. Usually, a small number of cells (<50,000, some authors even mention just 10,000 cells) is sufficient for analysis. Its major advantage is the ability to apply/modify it for almost all cell types and thus tissues that can be divided into individual cells. This capability allows for detecting DNA damage in tissues that are the first to come into contact with carcinogens. Additionally, the method's simplicity, speed, cost-effectiveness, and sensitivity in detecting even minor DNA damage are noteworthy.
As part of the CORE FACILITIES project, a laboratory for testing the genotoxic potential of various substances (factors) under in vitro, in vivo, or epidemiological study conditions has been newly equipped at the Faculty of Medicine in Hradec Králové. The laboratory features a biohazard box with laminar flow, an incubator with controlled atmosphere, a refrigerated centrifuge, a thermal block, and a fluorescence microscope with a CCD camera and software that enables the measurement of selected parameters during CA analysis.
Authors: prof. Ing. Zdeněk Fiala, CSc., MUDr. Andrea Málková, Ph.D.